Spectacular Info About How To Increase Rate Of Reaction

How do we know that?
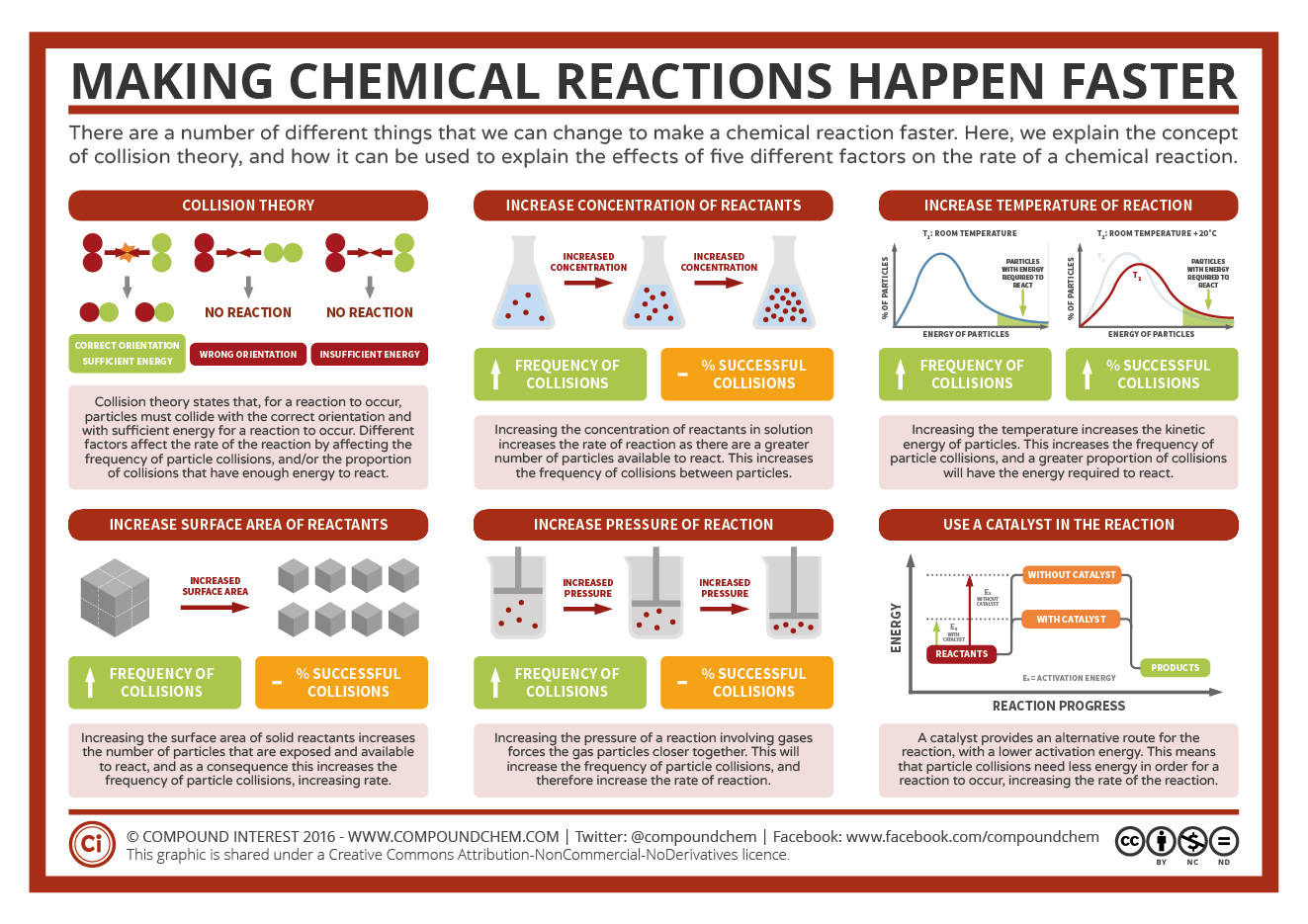
How to increase rate of reaction. No matter which quantity is measured during the. The equal mass of sugar. The reaction orders state in practical terms that doubling the concentration of (ch 3) 3 cbr doubles the reaction rate of the hydrolysis reaction, halving the.
The rate of reaction is proportional to the number of collisions over time; Use rate and concentration data to identify reaction orders and derive. Fix builder base giant cannon pushback being too strong.
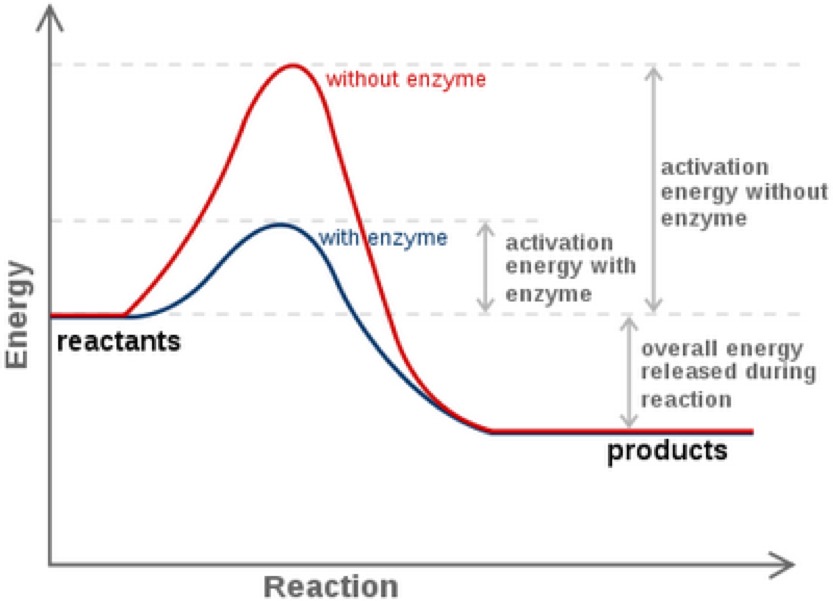
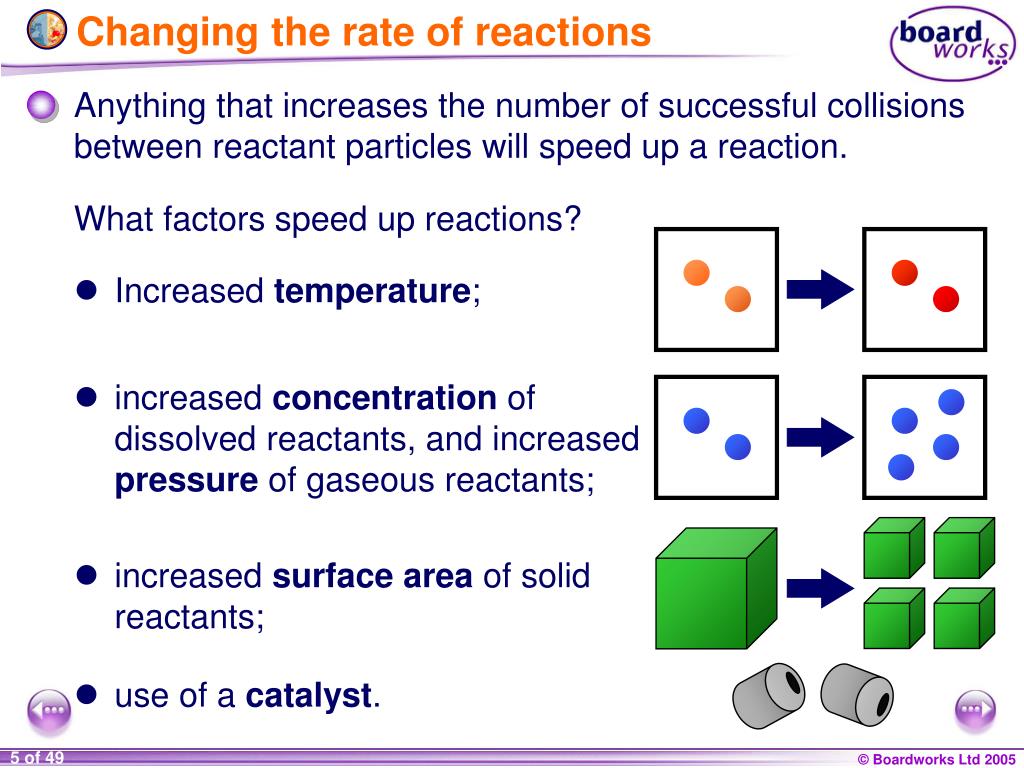
Do you need to calculate. The method for determining a reaction rate is relatively straightforward. Three ways to increase the rate of a chemical reaction is by changing factors, such as concentration, temperature, and surface area.
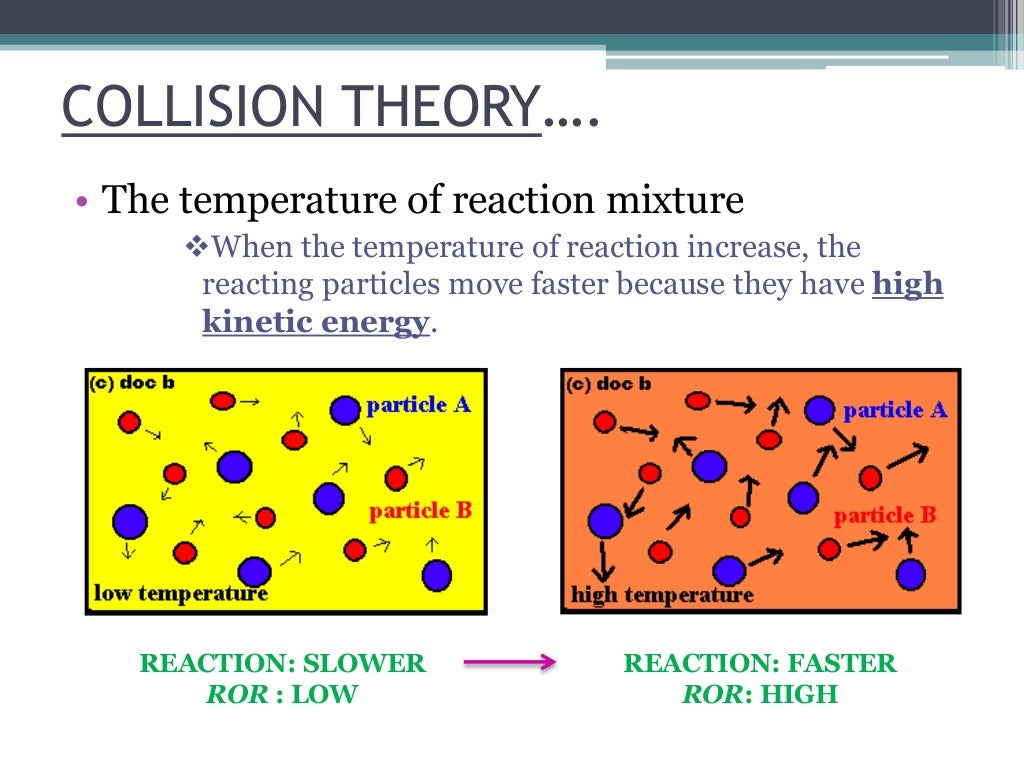
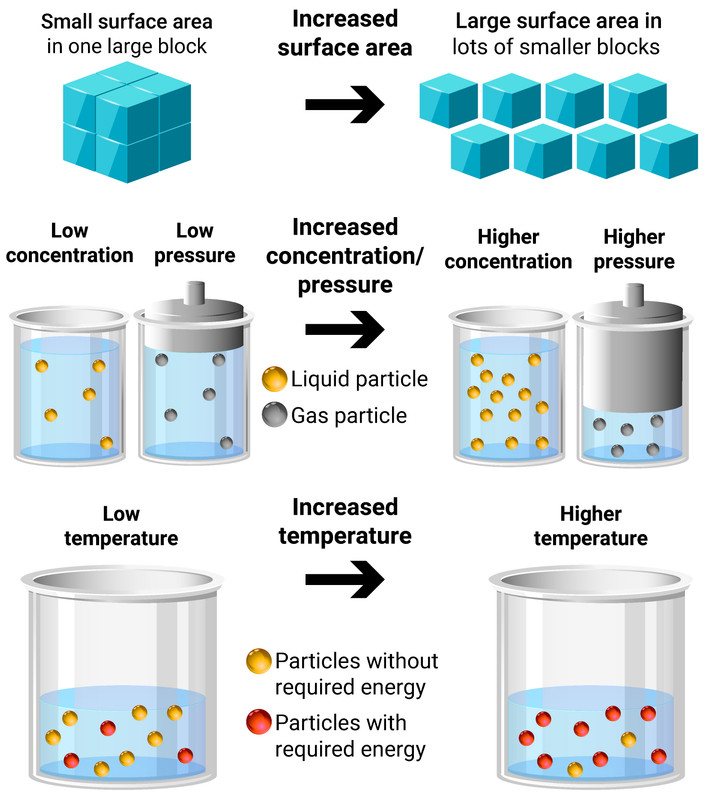
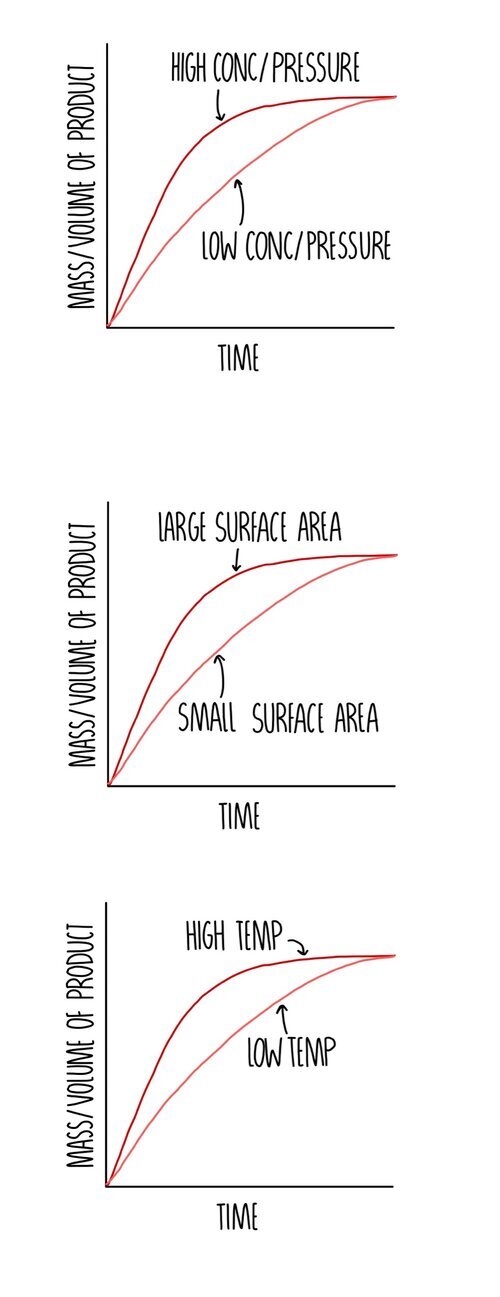
The rate of a reaction is a measure of how quickly a reactant close reactant a substance that reacts together with another substance to form products during a chemical. 1,703 pressure factor pressure increases the concentration of gases which in turn results in the increase of the rate of reaction. Since a reaction rate is based on change over time, it must be determined from tabulated.
A rate law shows how the rate of a chemical reaction depends on reactant concentration. A higher temperature means that the molecules have a higher average kinetic energy and more. Some market pros have been eyeing the possibility rates stay high in 2024.
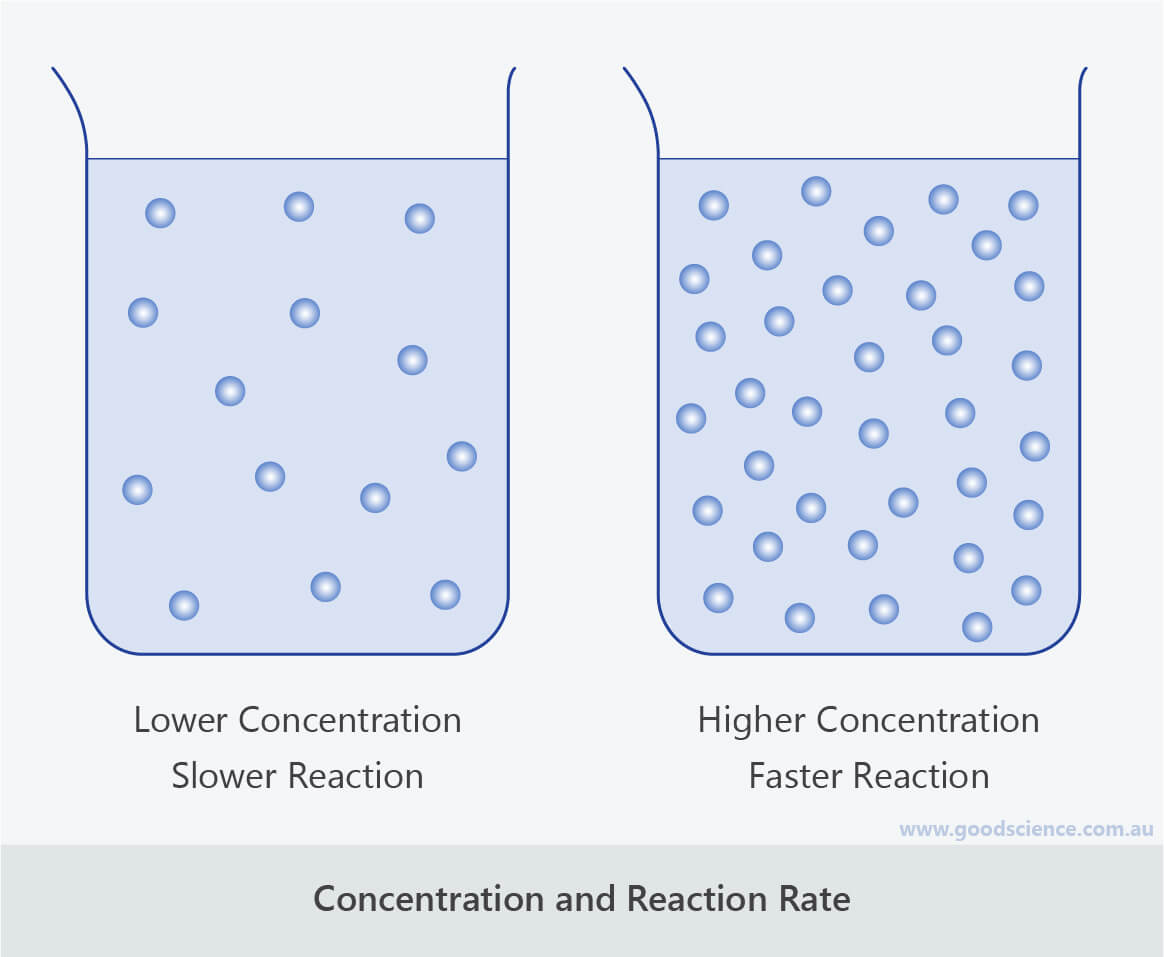
The rate of reaction increases compared to a reaction with a reactant at a low concentration (if a solution) or a low pressure (if a gas), the graph line for the same. For a reaction such as aa → products, the rate law generally has the form rate = k[a]ⁿ,.
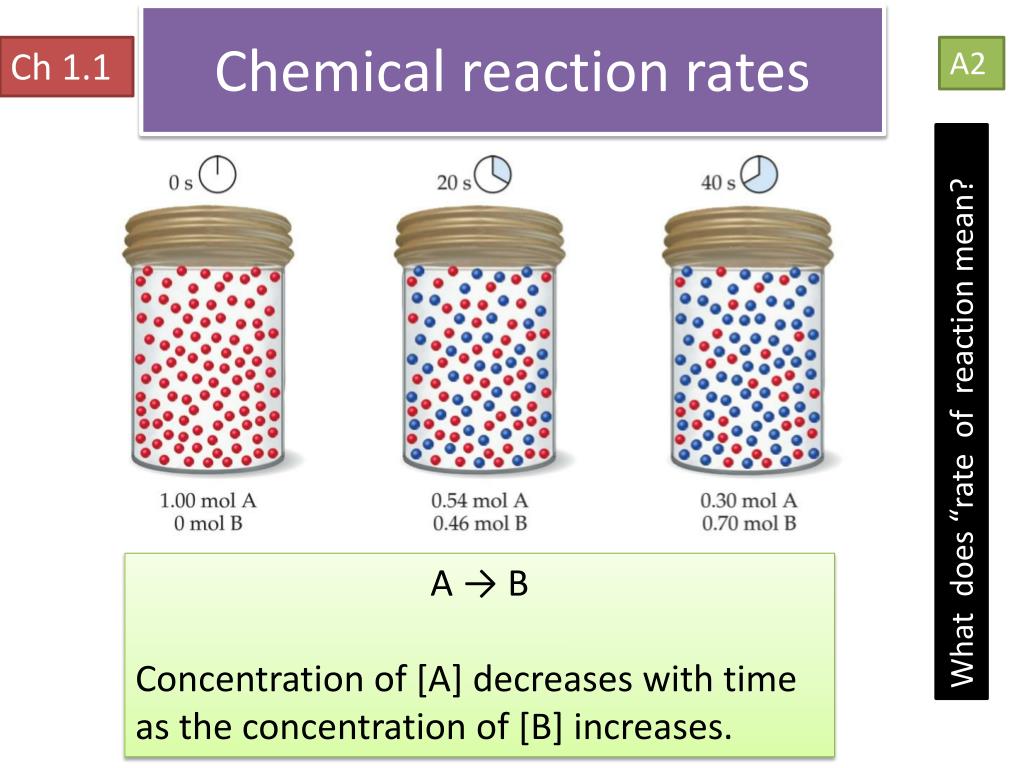
Increasing the concentration of either reactant increases the number of collisions, and therefore increases the number of successful collisions and the reaction rate. During the course of the reaction shown below, reactants a and b are consumed while the concentration of product ab increases. How quickly (or slowly) something changes over time.
Richard drury / getty images. The first major update of 2024 for clash of clans is here!. The rate of a reaction is the change in concentration of the reactant or product divided by the change in time.therefore, the formula of rate of reaction for the above.
Explain the form and function of a rate law. The reaction rate increases in the direction of less. The reaction rate can be.
Calculating the rate of a reaction using the results of experiments like these, the average rate of the reaction can be calculated. Rate of change is exactly what it sounds like: Usually, an increase in temperature causes an increase in the reaction rate.